The three mammalian isoforms of TGF-β, namely TGF-β1, β2, and β3, activate the same receptor and provoke similar biological responses. These versatile cytokines oversee cell proliferation, growth, differentiation, and motility, along with orchestrating the synthesis and placement of the extracellular matrix. They play vital roles in various physiological activities like embryogenesis, tissue restructuring, and wound healing. Typically, they are secreted in latent complexes, stored at cell surfaces and within the extracellular matrix.
For the biologically active TGF-β isoform to emerge from its latent state, the complex undergoes proteolytic processing or experiences conformational shifts induced by proteins like thrombospondin-1. While the precise role of TGF-β3 remains unclear, its expression pattern hints at involvement in regulating certain developmental processes.
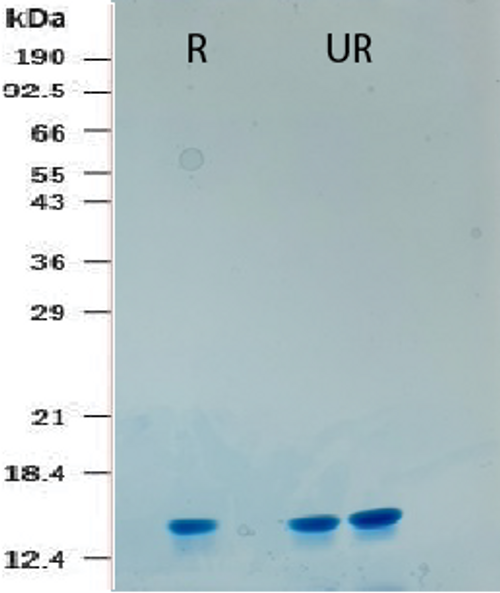
Recombinant Human TGF-β3 comprises two identical 112 amino acid polypeptide chains connected by a single disulfide bond, weighing 25.0 kDa.
|
Product Specifications
|
|
| Species | Human |
| Published species |
Amphibian, Human, Mouse, Rat
|
| Expression System | E. coli |
| Amino acid sequence |
ALDTNYCFRN LEENCCVRPL YIDFRQDLGW KWVHEPKGYY ANFCSGPCPY LRSADTTHST VLGLYNTLNP EASASPCCVP QDLEPLTILY YVGRTPKVEQ LSNMVVKSCK CS
|
| Molecular weight | 25 kDa |
| Class | Recombinant |
| Type | Protein |
| Purity |
≥ 98% by SDS-PAGE gel and HPLC analyses.
|
| Endotoxin concentration | <0.1 EU/µg |
| Activity |
Determined by TGF-beta3's ability to inhibit the mouse IL-4-dependent proliferation of mouse HT-2 cells. The expected ED50 is ≥ 0.05 ng/ml, corresponding to a specific activity of ≥ 2 x 10^7 units/mg.
|
| Conjugate | Unconjugated |
| Form | Lyophilized |
| Contains |
no preservative
|
| Storage conditions | -20°C |
CAUTION
For Research Use Only. Not for use in diagnostic procedures.